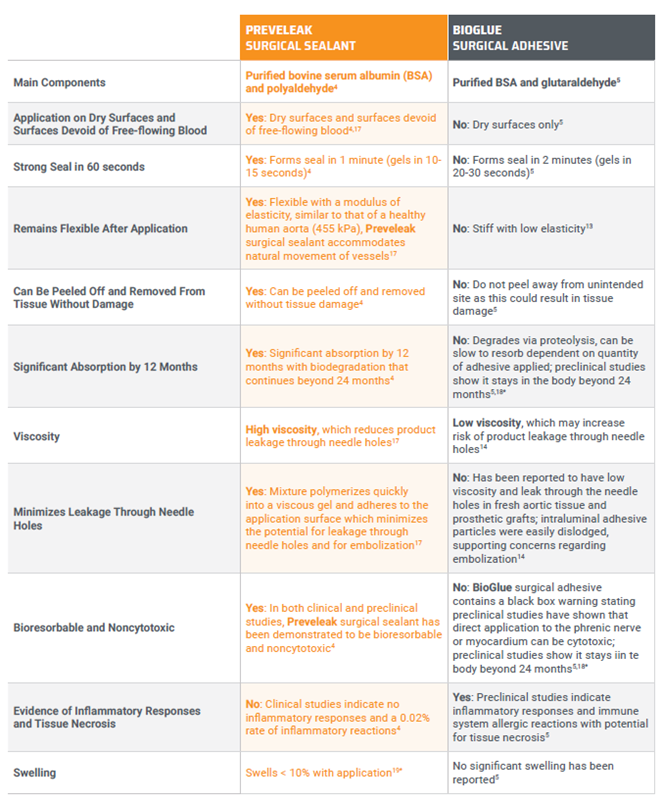
Preveleak Surgical Sealant and Bioglue Surgical Adhesive
O.R. Insights Blog
PREVELEAK Surgical Sealant and BIOGLUE Surgical Adhesive: The Use of Surgical Sealants and Adhesives in Cardiac Surgery
The control of intraoperative and postoperative bleeding is essential in the practice of cardiovascular surgery because reoperation and transfusion of blood products are both associated with poor outcomes.1 Managing the bleeding site with sutures and staples is often the first line of defense if the source of bleeding can be staunched surgically.2 However, surface area, proximity to surrounding structures, patient pathophysiology and source may limit the surgeon’s ability to gain control of bleeding using traditional methods.3
Over the past few decades, sealants and adhesives have been widely adopted to reinforce tissue and seal suture lines in cardiovascular surgery. 2 Among these, Preveleak Surgical Sealant is indicated specifically for adjunctive sealing in cardiovascular surgery,4 while BioGlue Surgical Adhesive is commonly used as an adjunctive tissue adhesive in cardiac surgery.5 As a medical professional, understanding the key differences between these two products is crucial for making informed decisions tailored to the specific needs of each surgical scenario. Here, Michael P. Salna, MD, MBA†, provides an overview of both products empowering you with the knowledge needed to elevate your surgical practice.
Please see Preveleak Surgical Sealant Indications and Important Risk Information to follow.
Introduction
Bleeding continues to be a significant complication of cardiac surgical procedures and effective management is important to prevent serious postoperative comorbidity or fatal consequences as well as adverse physiological effects associated with blood loss and transfusion. 2 Closing the bleeding site with sutures is often the first line of defense if the source of the bleeding can be stopped surgically.2 Sealing at the suture line is critical for patients undergoing cardiac surgical procedures, as many are at risk for poor hemostasis.2
Most cardiac procedures are performed using a cardiopulmonary bypass machine.6 The blood-plastic interface within this extracorporeal circulation, however, potentiates inflammation and coagulopathy for the patient.6 In aortic surgery, suture-line hemostasis is paramount. Failure to achieve hemostasis at anastomoses, particularly in fragile tissue, may lead to additional operative time, transfusion requirements, and possibly reoperation for bleeding.7 The introduction of advanced surgical sealants and adhesives, such as Preveleak Surgical Sealant and BioGlue Surgical Adhesive, have improved the ability to manage bleeding and reinforce fragile tissues.8,9 Understanding the differences between these products is essential for surgeons to make informed decisions tailored to specific clinical scenarios.
What is PREVELEAK Surgical Sealant?
Preveleak Surgical Sealant is a sealant developed to seal suture holes formed during surgical repair of the circulatory system and to reinforce sutured anastomoses.4 When applied, it creates an elastic biocompatible gel that seals suture holes or gaps formed in anastomoses between synthetic grafts or patches and native vessels. 4
Preveleak Surgical Sealant adheres to the native tissues as well as synthetic materials, including polytetrafluoroethylene (PTFE) and Dacron grafts, and facilitates sealing along closure lines.4 After application, it is a natural golden color and stays soft and flexible.4
Animal studies showed significant absorption by 12 months with biodegradation that continues beyond 24 months. 4 (Figure 1)
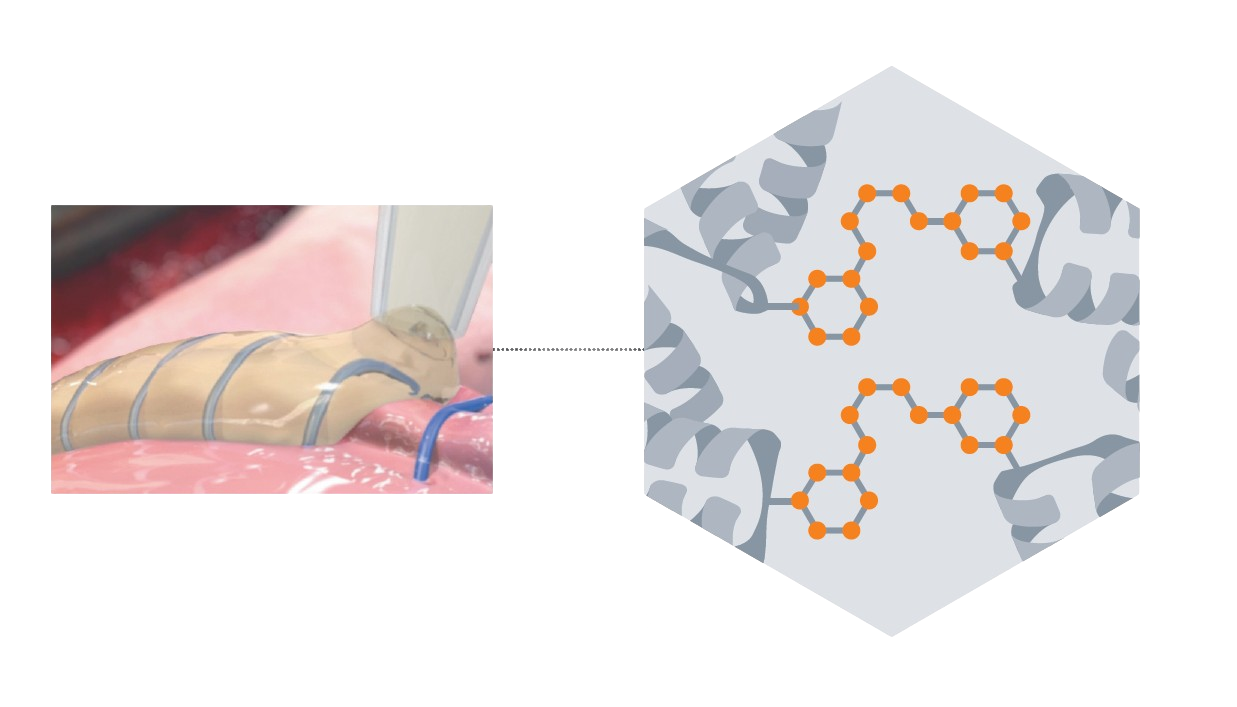
Figure 1. Application of Preveleak Surgical Sealant. Preveleak Surgical Sealant has a distinct composition that includes a propriety polyaldehyde crosslinking agent. 4 (Images property of Baxter)
What is the Indication?
Preveleak Surgical Sealant is indicated for use in vascular and cardiac reconstructions (excluding application to arterial and venous grafts used in coronary artery bypass graft surgery) to achieve adjunctive hemostasis by mechanically sealing areas of potential leakage. 4
Selected Risk Information for Preveleak Surgical Sealant: Do not use in patients with known allergies to materials of bovine or shellfish origin.4